LSPedia 的全球序列化系列
序列化不是一刀切的。由于美国、欧盟、亚洲和中东的规定各不相同,公司必须应对复杂的要求网络。您为全球合规做好准备了吗?
.avif)
欢迎来到 LspEdia,在这里,创新与奉献精神相结合。
如果你热衷于有所作为并在协作环境中茁壮成长,LspEdia 就是你的不二之选。

序列化不是一刀切的。由于美国、欧盟、亚洲和中东的规定各不相同,公司必须应对复杂的要求网络。您为全球合规做好准备了吗?
.avif)
如果你热衷于有所作为并在协作环境中茁壮成长,LspEdia 就是你的不二之选。

在南部非洲,药品法规正在逐步演变,以增强整个药品供应链的可追溯性、安全性和质量保证。该地区的许多国家正在实施越来越复杂的监管框架,以打击假冒药品,并通过改善供应链的完整性来确保患者安全,其中许多国家都参与了南部非洲发展共同体(SADC)。 南共体区域由15个成员国组成(安哥拉、博茨瓦纳、刚果民主共和国、莱索托、毛里求斯、马达加斯加、马拉维、莫桑比克、纳米比亚、塞舌尔、南非、斯威士兰和坦桑尼亚联合共和国(大陆和桑给巴尔)。 来源-https://tis.sadc.int/english/sarn/about-sarn/
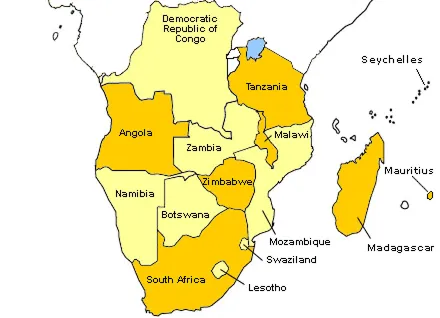
南部非洲国家正在通过Zazibona等举措共同努力调整其药品法规,其中包括赞比亚、津巴布韦、博茨瓦纳、纳米比亚、莫桑比克和坦桑尼亚。这种区域方法可以实现资源共享、联合产品审查和协调良好的生产规范(GMP)检查,从而在参与国之间创造更加统一的监管环境。
该地区正在逐步采用药品序列化要求,不同国家的实施阶段各不相同。赞比亚率先正在开发基于GS1的系统,该系统要求全球贸易项目号(GTIN)、批号、有效期和以二维DataMatrix代码编码的唯一序列号。这样可以在整个供应链中进行精确的产品识别和跟踪。
在南部非洲国家销售的所有药品必须显示标准化的书面信息,包括产品名称、活性成分、剂型、强度、批号、有效期和制造商详细信息。这些要求符合南部非洲发展共同体的指导方针,并确保整个地区的产品标签一致,从而提高药物安全和正确使用。
南部非洲国家采用了不同的时间表来实施可追溯系统,这反映了监管能力和基础设施的差异。尽管赞比亚和南非取得了重大进展,但博茨瓦纳等其他国家正在制定监管框架和编码标准。包括纳米比亚、津巴布韦、莫桑比克和坦桑尼亚在内的国家在建立国家体系的同时参与了ZaziBona等区域举措。
Our team of compliance experts possesses in-depth knowledge of iTS and Turkish pharmaceutical regulations. From serialization strategies to data reporting, we guide your organization through the intricacies of compliance, ensuring a smooth and informed journey.
Leverage our state-of-the-art compliance solution, designed to integrate seamlessly with iTS requirements. Our technology ensures accuracy, efficiency, and real-time visibility, empowering your organization to meet and exceed compliance expectations.
Compliance is not just about meeting today's standards; it's about preparing for the future. Our solution is designed to evolve with regulatory changes, ensuring that your organization remains at the forefront of compliance in the dynamic Turkish pharmaceutical landscape.
We believe in transparent collaboration. Partner with us, and we'll work closely with your team to implement and maintain compliance measures. Our goal is to build a resilient, compliant, and future-ready pharmaceutical supply chain together.


驾驭复杂的南部非洲药品法规格局需要专业知识和适应性技术解决方案。我们的平台提供针对每个南部非洲市场的特定要求量身定制的全面合规解决方案。从实施符合 GS1 的序列化系统到管理区域报告要求,我们的专业知识确保制药公司能够保持无缝运营,同时实现整个南部非洲地区的全面监管合规性。 我们的解决方案解决了该市场的独特挑战,包括不同的实施时间表、区域协调举措和国别报告要求。通过与我们合作,制药公司在应对这些不断变化的法规方面获得了战略优势,同时为整个南部非洲更安全、更透明的药物供应链做出了贡献。




We invite you to learn more about how LSPedia can support your compliance and supply chain needs. For more information about our solutions, partnership opportunities, or to speak with one of our experts.
Stay connected with us on social media and subscribe to our newsletter to receive the latest updates, insights, and innovations from LSPedia.